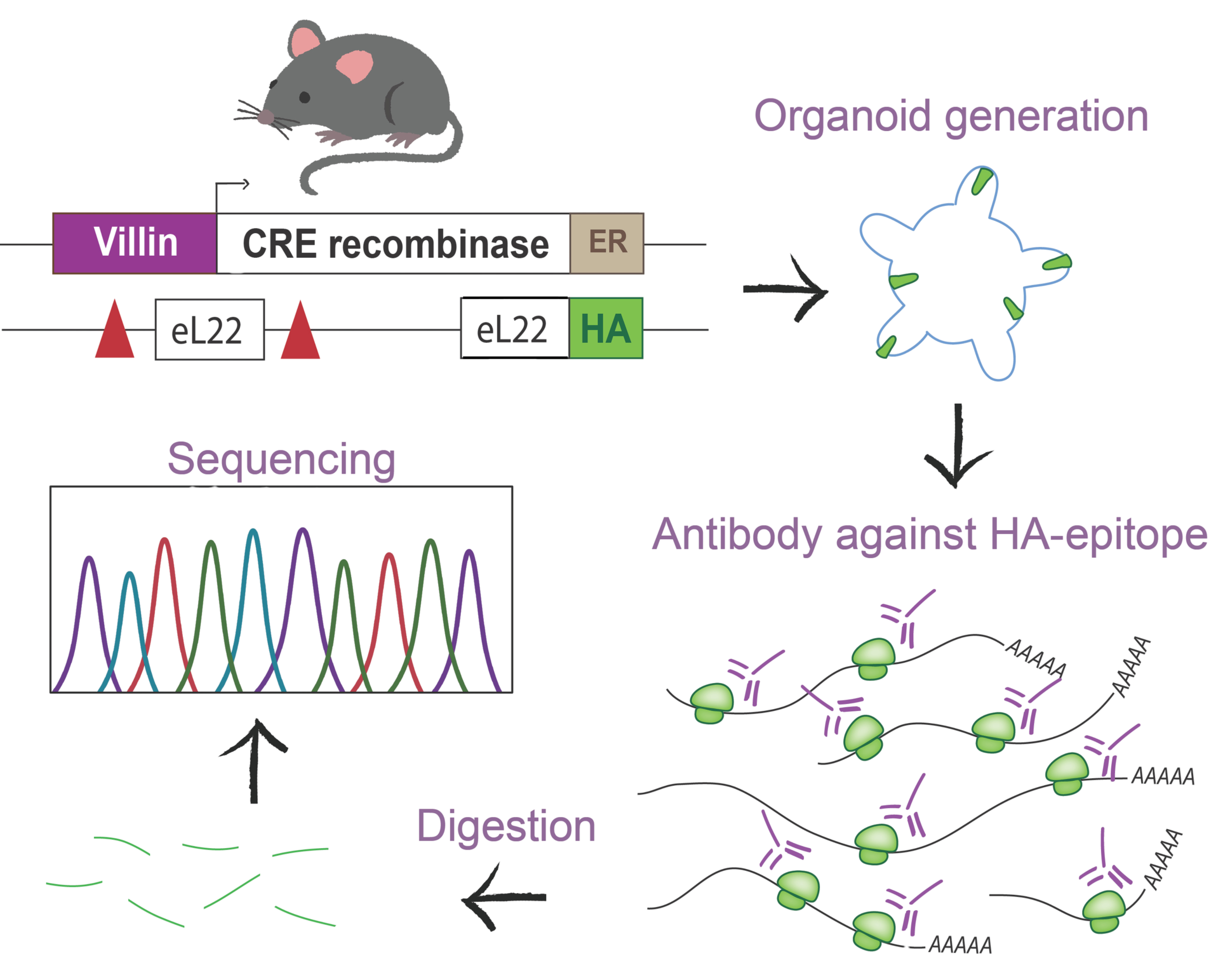
Silva Lab at UMC Utrecht
Decoding Cell Fate Through RNA Translation
How ribosomes shape cell metabolism, identity and plasticity
The ability of cells to make fate decisions - whether to proliferate, differentiate, or remain quiescent - lies at the core of development. Increasing evidence highlights that these decisions are influenced by cellular metabolism, yet the molecular mechanisms that connect metabolic states to cell fate remain poorly understood.
At the Silva Lab, we study how ribosomes act as regulators of metabolic and translational information, influencing cell behavior and fate. We are particularly interested in how spatial control of translation, particularly near and within mitochondria, can shape the metabolic programs that guide cell behavior. By uncovering how ribosome positioning governs mitochondrial function, our work aims to reveal new principles that underlie cell fate plasticity, regeneration, and disease.
Our goal is to redefine how we understand translation: not just as a process, but as a spatially organized system that governs cell identity and plasticity.
Research
Our research explores how translation and metabolism are integrated to control cell fate. We focus on three central questions that connect ribosome biology, mitochondrial function, and stem cell identity.
How do ribosomes respond to stress, and why does that matter?
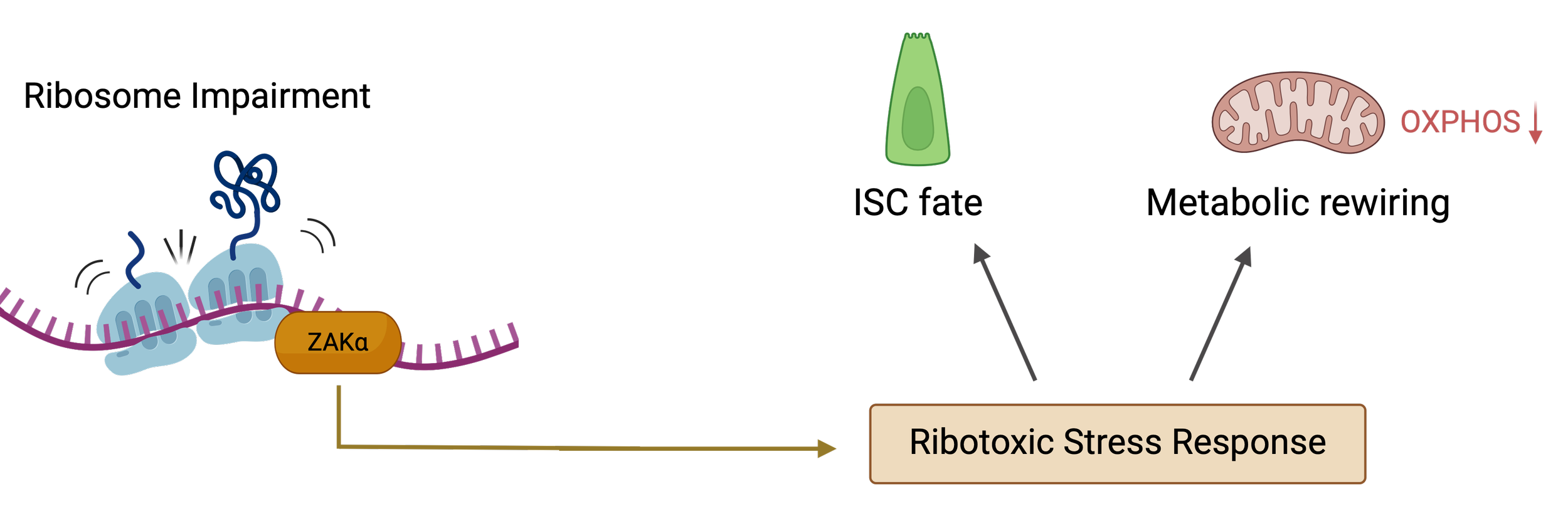
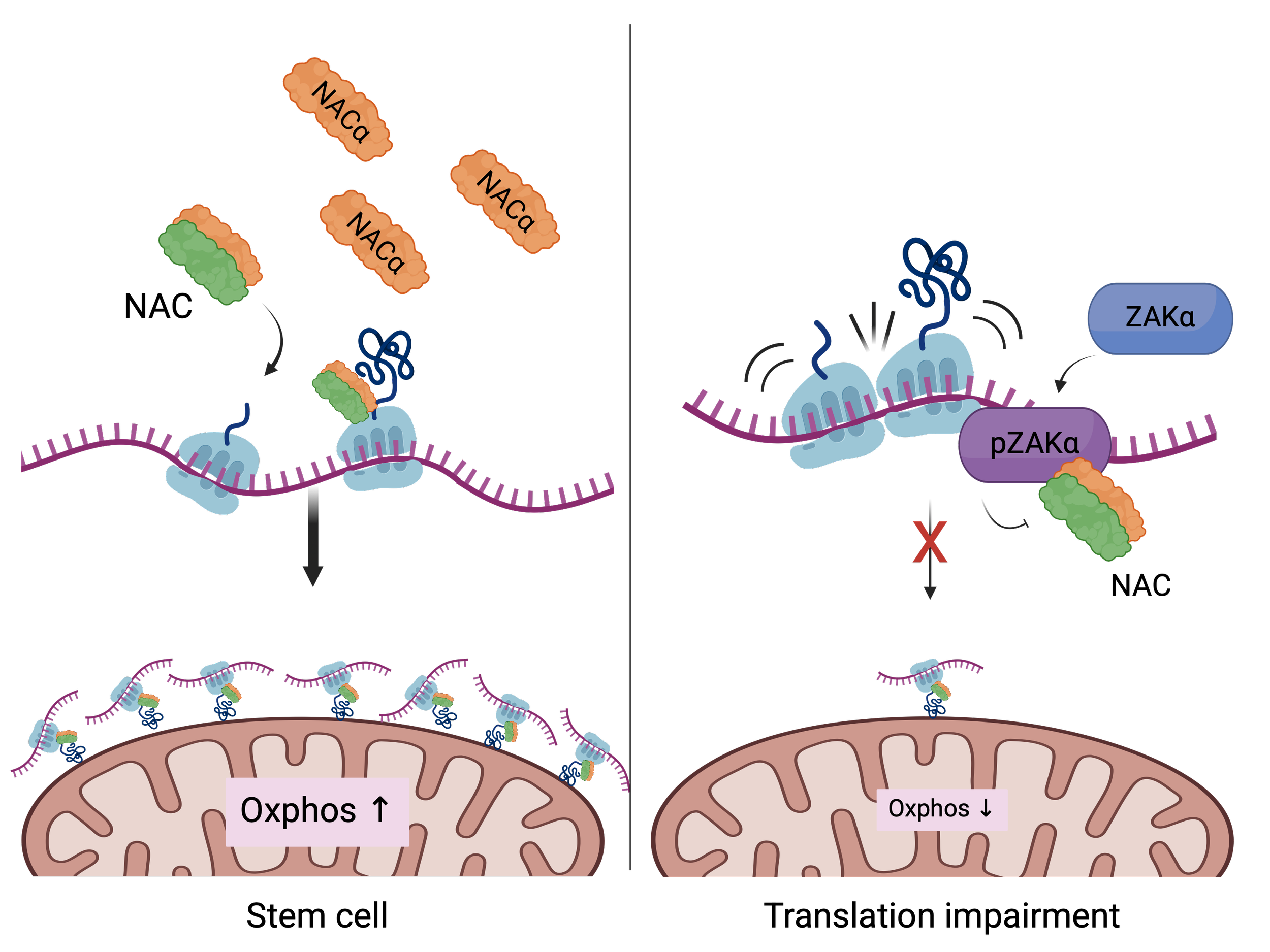
Cells frequently face conditions that impair protein synthesis, such as upon nutrient limitation or drug-induced stress. While translation slowdown is often seen as a passive consequence, our research shows it's also an active regulatory signal.
We discovered that translational stress in intestinal stem cells activates ZAKα, a ribotoxic stress-responsive kinase. This activation triggers a metabolic shift from OXPHOS to glycolysis, pushing adult stem cells into a fetal-like state that is more plastic and resistant to damage.
This finding reframes ribosomes as sensors and effectors of cell fate under stress. We are now investigating how this pathway is regulated, how it interfaces with other translation control factors, and how it influences long-term stem cell behavior.
2. How does ribosome positioning influence metabolism and cell fate?
Translation is not only regulated by what is translated, but also where it happens in the cell. We uncovered that ribosomes are actively repositioned within the cytoplasm to meet the cell’s metabolic needs.
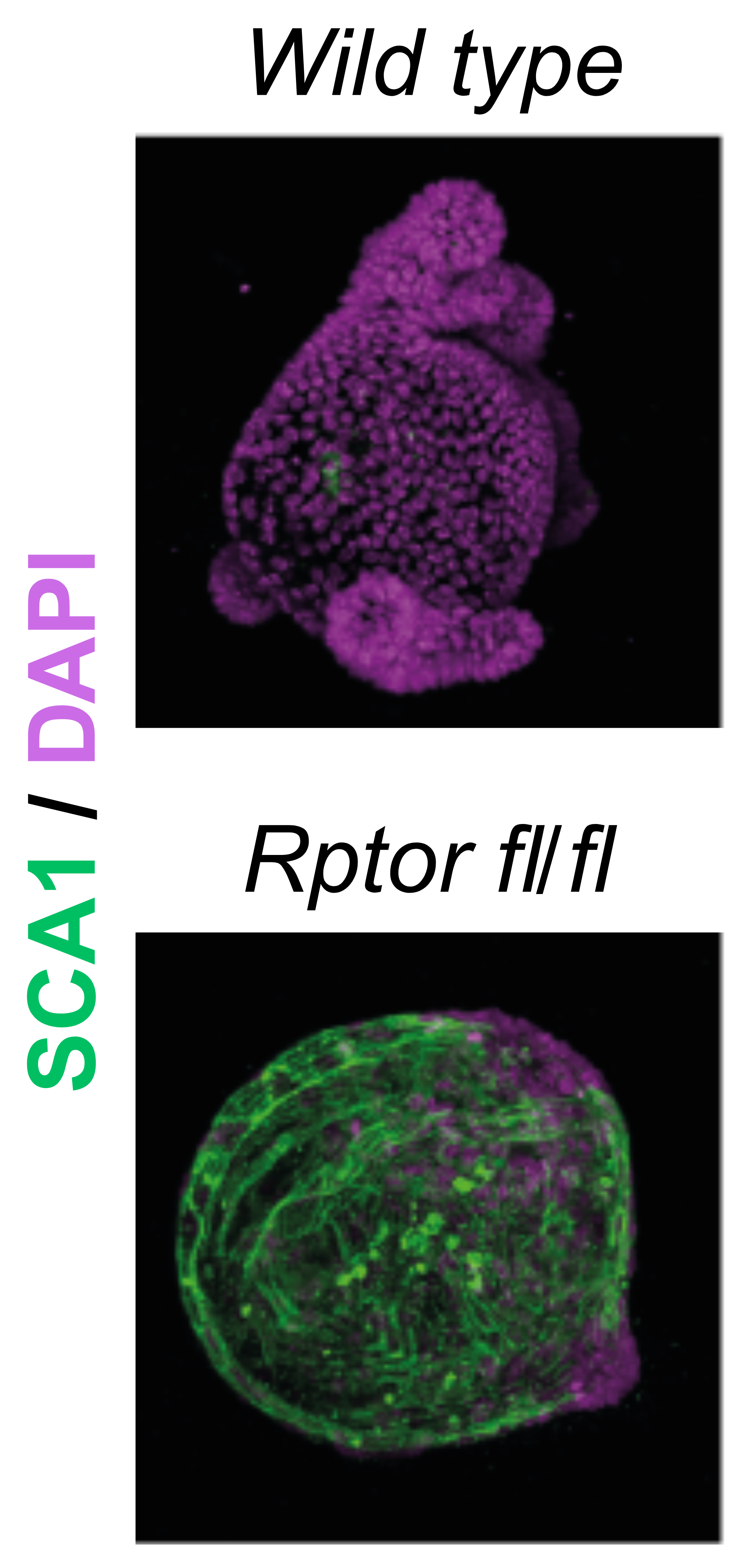
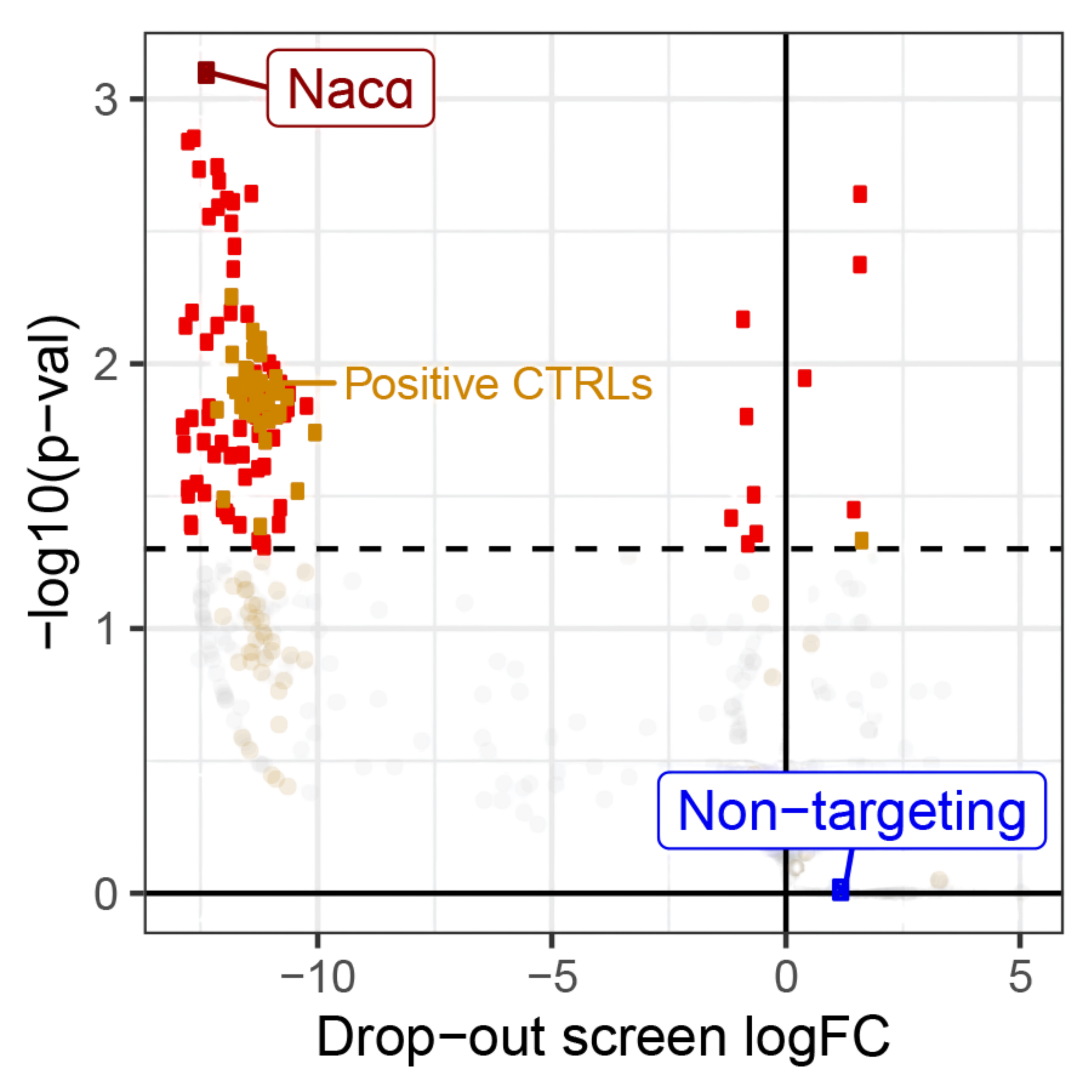
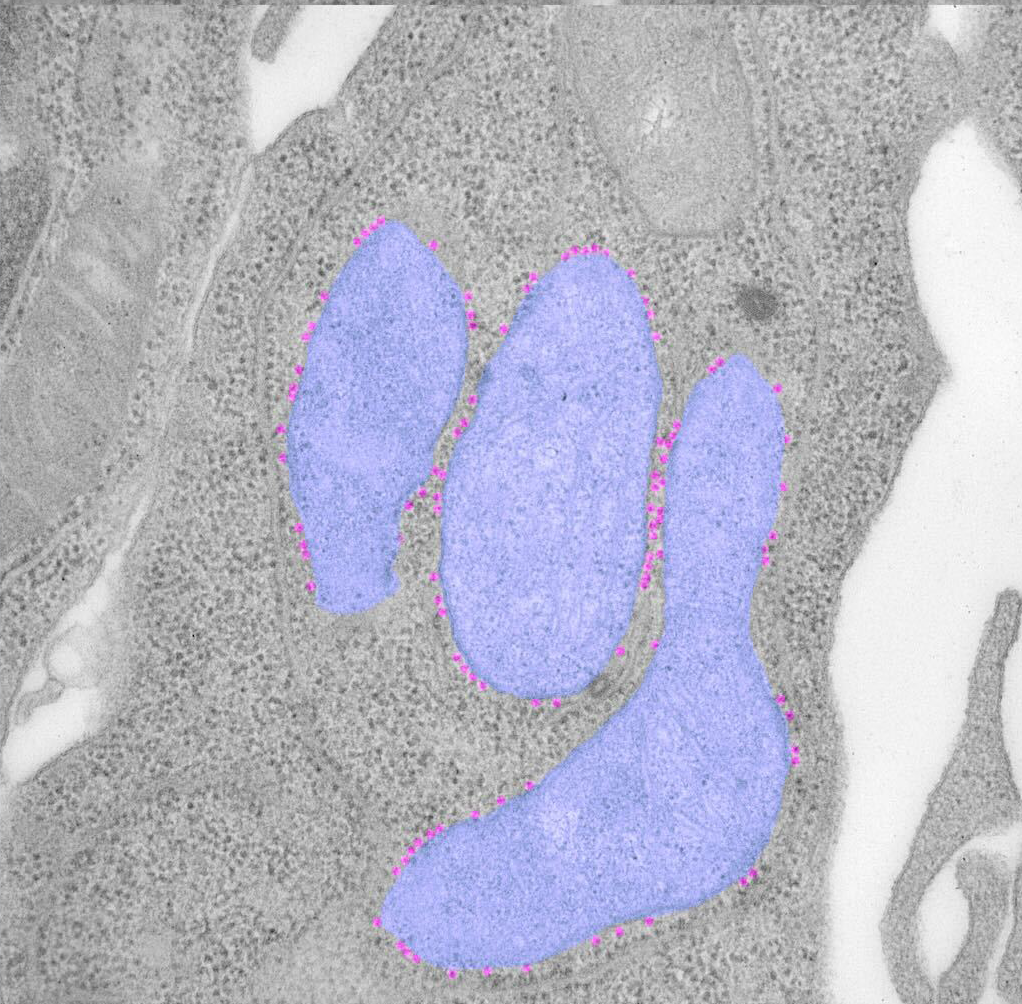
In intestinal stem cells, we found that a subset of ribosomes localize to the mitochondrial surface, where they facilitate the import of nuclear-encoded mitochondrial proteins. This spatial targeting, guided by the chaperone NAC , helps sustain high OXPHOS activity, which is critical for stem cell maintenance.
When this localization is disrupted by stress, or cell differentiation, mitochondrial function drops, and cells begin to reprogram. Our current work is exploring how this spatial control is regulated, and whether other players besides NAC are involved in this process.
3. How do ribosomes expand our view of the cellular proteome?
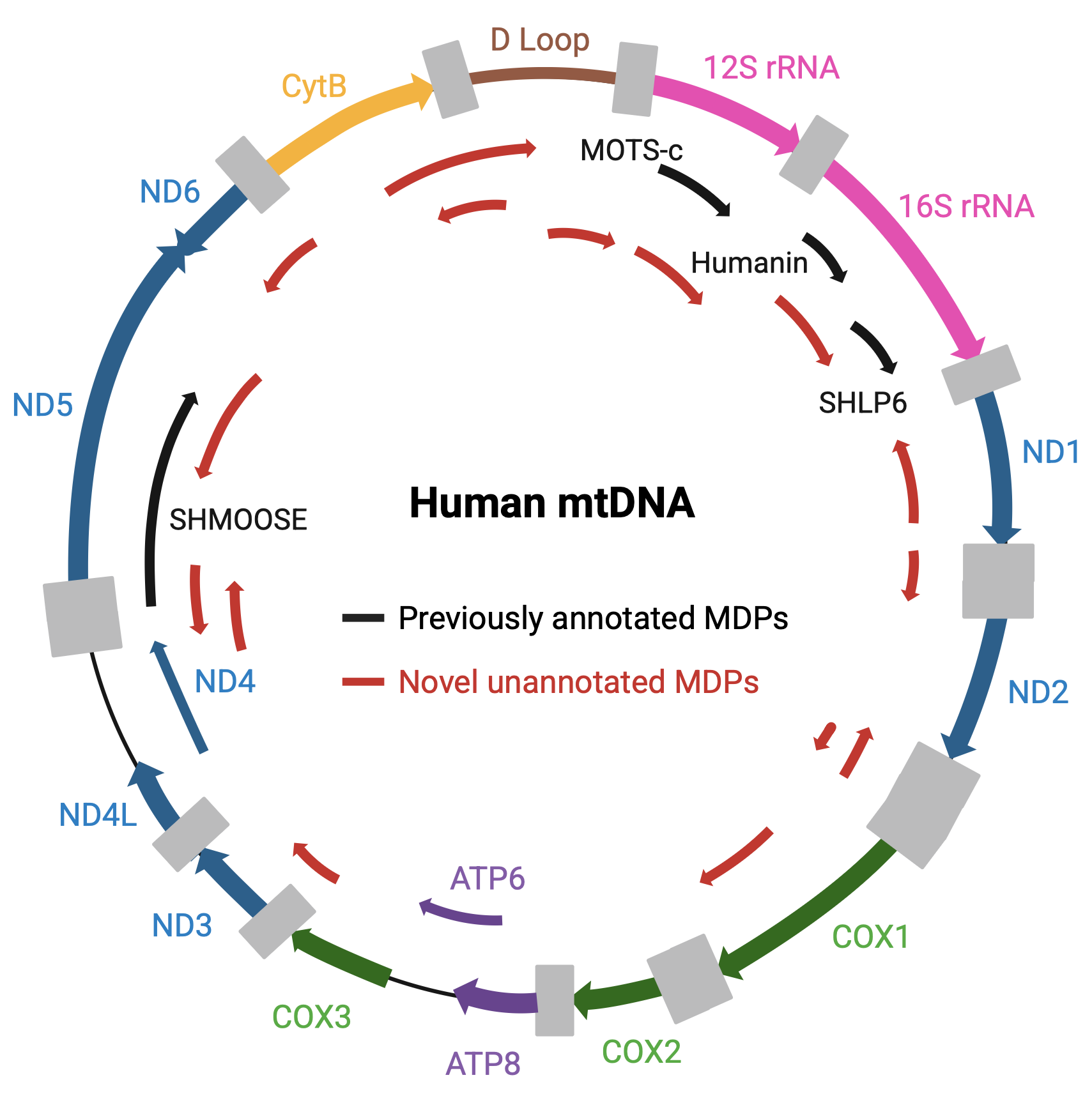
Advances in ribosome profiling have revealed that our understanding of the proteome is far from complete. Beyond the well-annotated set of canonical proteins lies the dark proteome, a growing collection of previously overlooked translation products, often encoded by small open reading frames (sORFs) hidden in regions once dismissed as non-coding.
These newly discovered peptides are frequently short, dynamic, and tightly regulated. Many respond to environmental cues or cellular stress, suggesting they play key roles in modulating metabolism, signaling pathways, or cell identity. Yet, their functions remain largely unexplored.
Our lab is particularly interested in how the mitochondrial genome contributes to this emerging layer of regulation. Despite being compact, mitochondria retain their own genetic code and translation machinery.
By charting this uncharacterized space, we hope to uncover new regulatory molecules that link mitochondrial activity to cell fate decisions, offering fresh insight into how cells adapt to stress and mediate plasticity.
Team
What I study: I’m fascinated by the idea that ribosomes are not passive machines but active regulators of cellular identity. By changing their position in space and responding to stress or developmental cues, they help shape how a cell senses, adapts, and ultimately decides what to become. My work focuses on uncovering how the spatial organization of translation influences mitochondrial function, metabolic rewiring, and stem cell fate decisions.
What I did before this: Postdoc work on ribosome dynamics in intestinal stem cells (Faller lab, NKI Amsterdam, NL). PhD in mitochondrial translation, metabolism and stem cell homeostasis (Stracker lab, IRB Barcelona, ES). MSc in cancer biomarkers using GWAS (Medeiros lab, IPO Porto, PT). BSc in Applied Biology (University of Minho, PT).
What I love outside the lab: Live music, jigsaw puzzles, swimming and playing Dixit.
Go-to track while pipetting: Summon the Fire by The Comet is Coming
Joana Silva
(she/her)
Group Leader
Alumni / Previously mentored
Sofia Ramalho (co-supervised by William Faller; based at the Netherlands Cancer Institute; 2019-2025) - currently Postdoc at the Bekker-Jensen lab in the University of Copenhagen - Contact
Danielle Barum (co-supervised by William Faller; based at the Netherlands Cancer Institute; 2019-2021) - currently Healthcare Strategy Consultant at Axios International - Contact
Publications
2025
Kyei-Baffour ES, Bak J, Silva J, Faller WJ# and Alkan F#
Detecting ribosome collisions with differential rRNA fragment analysis in ribosome profiling data
NAR Genomics and Bioinformatics, May 2025. PMID: 40342836
Ramalho S, Alkan F, Prekovic S, Jastrzebski K, Barberà EP, Hoekman L, Altelaar M, de Heus C, Liv N, Rodríguez-Colman MJ, Yilmaz M, van der Kammen R, Fedry J, de Gooijer MC, Suijkerbuijk SJE, Faller WJ# and Silva J#
NAC regulates metabolism and cell fate in intestinal stem cells
Science Advances, January 2025. PMID: 39772672
2024
Dopler A, Alkan F, Malka Y, van der Kammen R, Hoefakker K, Taranto D, Kocabay N, Mimpen I, Ramirez C, Malzer E, Isaeva OI, Kerkhoff M, Gangaev A, Silva J, Ramalho S, Hoekman L, Altelaar M, Beijersbergen R, Akkari L, Yewdell JW, Kvistborg P and Faller WJ
P-stalk ribosomes act as master regulators of cytokine-mediated processes
Cell, November 2024. PMID: 39437780
Fedry J#, Silva J, Vanevic M, Fronik S, Mechulam Y, Schmitt E, des Georges A, Faller WJ and Förster F
Visualisation of translation reorganization upon persistent ribosome collision stress in mammalian cells
Molecular Cell, March 2024. PMID: 38340715
2023
Spaan CN, de Boer RJ, Smit WL, van der Meer JHM, van Roest M, Vermeulen JLM, Koelink PJ, Becker MAJ, Go S, Silva J, Faller WJ, van den Brink GR, Muncan V and Heijmans J
Grp78 is required for intestinal Kras-dependent glycolysis proliferation and adenomagenesis
Life Science Alliance, August 2023. PMID: 37643866
2022
Molennar TM, Malik M, Silva J, Liu NQ, Haarhuis JHI, Ambrosi C, Kwesi-Maliepaard EM, van Welsem T, Baubec T, Faller WJ and van Leeuwen F
The histone methyltransferase SETD2 negatively regulates cell size
Journal of Cell Science, October 2022. PMID: 36052643
Alkan F, Wilkins OG, Hernández-Pérez S, Ramalho S, Silva J, Ule J and Faller WJ
Identifying ribosome heterogeneity using ribosome profiling
Nucleic Acids Research, September 2022. PMID: 35687114
Silva J, Alkan F, Ramalho S, Snieckute G, Prekovic S, Garcia AK, Hernández-Pérez S, van der Kammen R, Barnum D, Hoekman L, Altelaar M, Zwart W, Suijkerbuijk SJE, Bekker-Jensen S and Faller WJ
Ribosome impairment regulates intestinal stem cell identity via ZAKa activation
Nature Communications, August 2022. PMID: 35918345
2021
Alkan F*, Silva J*, Barberà EP and Faller WJ
Ribo-ODDR: oligo design pipeline for experiment-specific rRNA depletion in Ribo-seq
Bioinformatics, September 2021. PMID: 33720291
Prekovic S, Schuurman K, Mayayo-Peralta I, Manjón AG, Buijs M, Yavuz S, Wellenstein MD, Barrera A, Monkhorst K, Huber A, Morris B, Lieftink C, Chalkiadakis T, Alkan F, Silva J, Győrffy B, Hoekman L, van den Broek B, Teunissen H, Debets DO, Severson T, Jonkers J, Reddy T, de Visser KE, Faller WJ, Beijersbergen R, Altelaar M, de Wit E, Medema R and Zwart W
Glucocorticoid receptor triggers a reversible drug-tolerant dormancy state with acquired therapeutic vulnerabilities in lung cancer
Nature Communications, July 2021. PMID: 34272384
Garcia TM, van Roest M, Vermeulen JLM, Meisner S, Smit WL, Silva J, Koelink PJ, Koster J, Faller WJ, Wildenberg ME, van Elburg RM, Muncan V and Renes IB
Early life antibiotics influence in vivo and in vitro mouse intestinal epithelium maturation and functioning
Cellular and Molecular Gastroenterology and Hepatology, June 2021. PMID: 34102314
2018
Silva J, Aivio S, Knobel PA, Bailey LJ, Casali A, Vinaixa M, Garcia-Cao I, Coyaud E, Jourdain AA, Pérez-Ferreros P, Rojas AM, Antolin-Fontes A, Samino-Gené S, Raught B, González-Reyes A, de Pouplana LR, Doherty AJ, Yanes O and Stracker TH
EXD2 governs germ stem cell homeostasis and lifespan by promoting mitoribosome integrity and translation
Nature Cell Biology, February 2018. PMID: 29335528
2014
Teixeira AL, Ferreira M, Silva J, Gomes M, Dias F, Santos JI, Maurício J, Lobo F and Medeiros R
Higher circulating expression levels of miR-221 associated with poor overall survival in renal cell carcinoma patients
Tumor Biology, May 2014. PMID: 24379138
Pereira C, Sousa H, Silva J, Brandão C, Elgueta-Karstegl C, Farrell PJ, Medeiros R and Dinis-Ribeiro M
-1995G allele increases the transcriptional activity of cyclooxygenase-2 gene (COX-2) in colon cancer cell lines
Molecular Carcinogenesis, February 2014. PMID: 23776069
2012
Silva J, Teixeira AL, Lobo F, Maurício J and Medeiros R
DNA repair system and prostate cancer progression: the role of NBS1 polymorphism (rs1805794)
DNA and Cell Biology, July 2012. PMID: 22413803
2011
Lima L, Silva J, Amaro T, Morais A, Lopes C, Medeiros R, Videira PA and Santos L
IL-4 and TNF-a polymorphisms are associated with risk of multiple superficial tumors or carcinoma in situ development
Urologia Internationalis, November 2011. PMID: 22104567
Fontenete S*, Silva J*, Teixeira AL, Ribeiro R, Bastos E, Pina F and Medeiros R
Controversies in using urine samples for prostate cancer detection: PSA and PCA3 expression analysis
International Brazilian Journal of Urology, November 2011. PMID: 22234006
News
01 July 2025
🎉 The Silva Lab officially launched on July 1st at the Center for Molecular Medicine (CMM) in Utrecht! We’re excited to start exploring how ribosomes shape metabolism and cell plasticity, and to build a collaborative, curious, and creative team.